EXTERNALLY FUNDED PROJECTS:
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
Participants
PI's: David Millhorn, Thomas Zawodzinski (CBE), Gregory Sedrick, Sandra Rosenthal, Barry Bruce (BCMB)
Other Info
Funding Agency: National Science Foundation - EPSCOR
Amount: $20,000,000
Duration: 8/15/2010 - 7/31/2015
Description:
None
∆ back to top
Participants
PI: Mark Dadmun (Chemistry)
Other Info
Funding Agency: National Science Foundation
Amount: $342,000
Duration: 9/1/2010 - 8/30/2013
Description:
In this research program, neutron scattering is utilized to determine the miscibility, phase diagram, phase-separated structure, interfacial characteristics, and vertical phase separation of conjugated polymer:fullerene thin film mixtures as a function of polymer and fullerene structure, thermal processing, surface structure, and solvent. Conjugated polymers (CPs) are chosen as the focus of this study as they are a promising class of materials for use in the conversion of solar energy to electricity. Unfortunately, current studies of CP based organic photovoltaics are very Edisonian, where most studies create the CP nanocomposite, measure its photovoltaic (PV) properties and then attempt to ascertain the relationship between the PV activity and morphology based on the observed morphological features. There exists no a priori control of the morphology. This research program is designed to supply thermodynamic information that will provide methods to control the formation of resultant CP:fullerene morphologies based on known surface and/or interfacial energies, and polymer:fullerene, polymer:solvent, and fullerene:solvent interactions. This knowledge will then be used to tune the interactions in the system to fabricate active layers with targeted structures. Correlation of PV activity to the fully characterized targeted morphology represents a paradigm shift in how the nanoscale morphology and photovoltaic activity are optimized in organic photovoltaics. Neutron scattering and reflectivity will be the primary tools in this project, as the large difference in neutron scattering length density between protonated polymers and fullerenes singularly allows the efficient and thorough characterization of the assembly, interfacial structure, morphology, and composition of polymer:fullerene systems.
∆ back to top
Participants
PI's: Bamin Khomami (CBE), Bill Dunne (Admin), Wesley Hines (Admin), Bill Hamel (MABE)
Other Info
Funding Agency: National Science Foundation
Amount: $1,946,521
Duration: 10/1/2010 - 9/30/2013
Description:
None
∆ back to top
Participants
PI's: Hanno Weitering. Bamin Khomami (CBE), Gerd Duscher (MSE), Mark Dadmun (Chemistry), Norman Manella
Other Info
Funding Agency: National Science Foundation
Amount: $661,200
Duration: 10/1/2010 - 9/30/2012
Description:
None
∆ back to top
Participants
PI: Bin Hu (MSE)
Other Info
Funding Agency: National Science Foundation
Amount: $360,000
Duration: 5/1/2011 - 4/30/2014
Description:
The project objective is to use magneto-optical measurements as effective experimental tools to investigate the critical parameters in controlling charge dissociation at donor-acceptor interfaces, charge transport in interpenetrating donor and acceptor networks, and charge collection at electrodes in organic solar cells. This project is proposed based on following two fundamental arguments. First, organic solar cells have shown significant improvements on photovoltaic efficiencies with the value exceeding 8%. This development brings an important research task: elucidating internal photovoltaic processes and their controlling parameters to provide critical understanding for further improvement of device efficiencies. Second, our recent studies have found that magneto-optical measurements: magnetic field effects of photocurrent and photoexcitation-assisted dielectric response can function as effective experimental tools to show the charge dissociation, transport, and collection in organic solar cells. Based on these two fundamental arguments, this project will use magneto-optical measurements to specifically investigate (i) the binding energies of electron-hole pairs at donor-acceptor interfaces, (ii) the electrical drifting in charge transport in interpenetrating donor and acceptor interfaces, and (iii) dielectric effects on charge collection at electrodes. The goal of this project is to elucidate the critical parameters in controlling internal photocurrent-generation processes for material engineering and device design to develop highly efficient organic solar cells.
∆ back to top
Participants
PI: Bamin Khomami (CBE)
Other Info
Funding Agency: Department of Energy
Amount: $600,086
Duration: 9/01/2009 - 8/31/2012
Description:
The purpose of this project is to quantify the interfacial transport of water into the most prevalent nuclear reprocessing solvent extractant, namely tri-butyl-phosphate (TBP), via massively parallel molecular dynamics simulations on the most powerful machines available for open research. Specifically, we will accomplish this objective by evolving the water/TBP/dodecane system up to 1 ms elapsed time, and validate the simulation results by direct comparison with experimentally measured water solubility in the organic phase. The significance of this effort is to demonstrate for the first time that the combination of emerging simulation tools and state-of-the-art supercomputers can provide quantitative information on par to experimental measurements for solvent extraction systems of relevance to the nuclear fuel cycle.
∆ back to top
Participants
PI: Gerd Duscher (MSE)
Other Info
Funding Agency: National Science Foundation
Amount: $494,521
Duration: 9/01/2009 - 8/31/2013
Description:
Silicon carbide is the most promising material for high power switches, as needed for electrical and hybrid vehicles. Also high power switches would enable a deregulated power grid which is necessary to accommodate the de-centrally generated energy of alternative and renewable sources.
Experimental work in the late 1990’s led to the conclusion that the problem is caused by a high density of interfacial defects, primarily caused by excess carbon. In the last 8 years, the co-PIs of this proposal have been at the forefront of research aiming to increase the mobility by reducing the density of interfacial defects or passivating them. The team developed, characterized, and systematized a nitridation process that reduces the interfacial defect density significantly andimproves electron mobilities by about one order of magnitude. The attainable mobilities are suitable for limited applications, but are still below the value required for the ultimate impact of this technology. The NO process has been patented, licensed by Cree, Inc. for the production of commercial electronics, and accepted world-wide in the SiC research community.
In the last 5 years, no further improvement of the mobility has been achieved ; even so the theoretical mobility of SiC is more than two orders of magnitude higher than achieved in state of the-art devices. The oxidation of SiC and the properties of the resulting SiC/SiO2 interface are at the core of the objective to develop SiC-based electronic devices for industrial and military applications that entail high power or high temperatures and impact energy conservation. We recently discovered a transition layer at this interface whose thickness scales directly with the mobility of the devices. With a synergistic study of the processing parameter, the mobility characterization, atomic structure determination with the scanning transmission electron microscopy and ab initio materials modeling. This work is also supported by Cree, Inc. the world leader in SiC devices.
∆ back to top
Participants
PI's: Mark Dadmun (Chemistry); Bin Hu (MSE), Bamin Khomami (CBE), Jimmy Mays (Chemistry)
Other Info
Funding Agency: National Science Foundation
Amount: $300,000
Duration: 9/01/2009 - 8/31/2012
Description:
Conjugated polymers (CPs) are a promising class of materials for use in the conversion of solar energy to electricity. For optimal performance in bulk heterojunction CPs, the morphology of the donor and acceptor materials must form percolating interpenetrating networks maximizing interfacial contacts w/ length scale of ~10 nm.
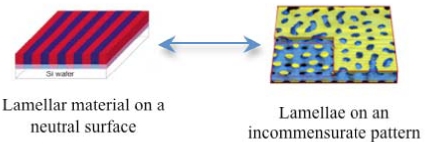
Currently, we lack the fundamental understanding to guide the formation of bulk heterojunctions to these targeted nanoscale morphologies. In this collaborative proposal, an understanding of the fundamental driving forces that govern the nanoscale self-assembly and interfaces in conjugated block copolymer (BCP) thin films will be developed in order to enable the rational design and fabrication of the targeted bicontinuous nanoscale morphologies. This will be realized by completing an interdisciplinary research program that will detail the thermodynamic driving forces that control the formation of a bicontinuous interconnected percolated morphology in a thin film of conjugated BCP with controlled rigidity on a surface that is patterned incommensurately to the periodicity of the diblock copolymer as well as the synthesis and thin film structure of conjugated diblock polymers that exhibit traditional diblock morphologies. Therefore upon completion, we will attain an understanding of the thermodynamics that control the assembly of these systems in thin films; enabling the reproducible creation of the desired bicontinuous interconnected morphologies with this structure, providing a transformative method to rationally design, tailor and fabricate nanoscale morphologies with exquisite control of size and thickness for CP systems.
∆ back to top
Participants
PI: Ramki Kalyanaraman (CBE)
Participants: Bamin Khomami (CBE), Chuck Melcher (SMRC), Mark Dadmun (Chemistry), Carl McHargue (CMP)
Other Info
Funding Agency: UT SARIF Equipment and Infrastructure Award
Amount: $24,250
Duration: 11/12/2009 - 6/30/2010
Description:
The main objective of this project is to develop composite and multilayered thin film materials with applications to energy harvesting, information processing, structural materials, and radiation detection. To enable this, capabilities to deposit multiple different materials, either in sequence or simultaneously, is essential. This multi-pocket e-beam system will permit a variety of inorganic materials to be deposited, including, metals, semiconductors, and ceramics.
∆ back to top
Participants
PI: Jimmy Mays (Chemistry)
Other Info
Funding Agency: Oak Ridge National Laboratory
Amount: $24,500
Duration: 1/1/2010 - 9/1/2010
Description:
None
∆ back to top
Participants
PI: Bin Hu (MSE)
Other Info
Funding Agency: National Science Foundation
Amount: $374,047
Duration: 2/1/2010 - 1/31/2013
Description:
None
∆ back to top
Participants
PI: Bamin Khomami (CBE)
Other Info
Funding Agency: Gibson Family Fellowship
Amount: $250,000
Duration: 2/1/2010 - 1/31/2015
Description:
None
∆ back to top
Participants
PI: Bamin Khomami (CBE)
Other Info
Funding Agency: Gibson Family Fellowship
Amount: $250,000
Duration: 2/1/2010 - 1/31/2015
Description:
None
∆ back to top
Participants
PI's: David Keffer (CBE), Paul Frymier (CBE), Bamin Khomami (CBE), Barry Bruce (BCMB), Claudia Rawn (MSE)
Other Info
Funding Agency: National Science Foundation
Amount: $2,941,396
Duration: 8/1/2008 - 7/31/2013
Description:
The STAIR program at the University of Tennessee is a new, interdisciplinary PhD program linking researchers in the following departments:
- Chemical and Biomolecular Engineering (CBE)
- Materials Science and Engineering (MSE)
- Biochemical, Cellular and Molecular Biology (BCMB)
- Chemistry (Chem)
- Civil and Environmental Engineering (CEE)
Sustainable Technology through Advanced Interdisciplinary Research (STAIR) is an NSF-funded IGERT program for graduate education at the University of Tennessee, Knoxville. The STAIR program provides an opportunity for talented scientists and engineers to earn a PhD inone of three areas in sustainable energy including production of hydrogen through biological pathways, discovery of nanoporous materials for hydrogen storage, and identifying structure/property relationships in hydrogen-based fuel cells.
Participants develop expertise in one of these three tasks and through an integrated curriculum, become knowledgeable in the broad range of research challenges for sustainable energy. Moreover, participants are exposed to the many social constraints that impact new energy technologies.
∆ back to top
Participants
PI's: David Keffer (CBE), Sandeep Agnihotri (CEE), Craig Barnes (Chemistry), Bob Compton (Chemistry), Claudia Rawn (MSE)
Other Info
Funding Agency: Federal Transit Administration
Amount: $380,267
Duration: 9/23/2008 - 12/21/2010
Description:
Hydrogen must be stored by safe and economical means for transport and distribution. The primary challenge is finding an adsorbent that will reversibly hold H2 at a high density (gravimetric (9 wt %) and volumetric density (70 g/L) capacities) at ambient temperature and pressure. The need for ambient temperature and pressure is driven by the overall sustainability argument that one cannot use more energy than the H2 contains in order to either pressurize it or cool it for storage. The storage material must also allow for rapid and controllable charging and discharging of hydrogen from the adsorbent. The objective of this work is to model the hydrogen adsorptive capacity of three new nanostructured adsorbents, currently being synthesized at the University of Tennessee: Metal Porphyrin Frameworks (MPFs), Decorated Carbon Fullerenes (DCFs) and Organometallic Grafted Silsesquioxanes (OGS). We perform molecular-level simulation of the adsorption and diffusion of hydrogen on these novel materials, in order to complement the experimental synthesis and characterization efforts already underway.
∆ back to top
Participants
PI's: Paul Frymier (CBE), Barry Bruce (BCMB)
Student Participants: Natalie Myers (MS, BCMB), Ifeyinwa Iwuchukwu (PhD, CBE), Ernest Iwuchukwu (MS, IE)
Other Info
Funding Agency: National Science Foundation
Amount: $90,000
Duration: 11/01/2008 - 10/31/2009
Description:
The NSF/SEERC-funded SPHERE (Sustainable Photosynthetic Hydrogen Evolution Research) Laboratory was founded to elucidate the processes that limit the rate of hydrogen production in a solar-powered nanoparticle that is built around photosystem I extracted from cyanobacteria.
We have shown that PSI-mediated hydrogen evolution using a bio-Pt composite system could potentially produce over 20 times the fuel energy per unit area of the current best biomass-to-energy solution (switchgrass-produced ethanol). Our current research program will enable an improvement on this accomplishment by making possible a highly engineered, kinetically enhanced, spectrally extended, photosynthetic hydrogen-evolving nanoparticle. Four specific tasks are required to accomplish this more sustainable and economically viable hydrogen-evolving system. These tasks are:
- creation of multiple subunit deletion mutants of photosystem I (PSI) from a thermophillic cyanobacterium,
- creation of fusion complexes between an appropriate hydrogenase enzyme and the multiple deleted subunits from PSI,
- creation of appropriate point-mutations in cytochrome c553 and the binding subunit in PSI, and
- creation of fusion complexes between PSI and phycobilisomes.
We are accomplishing this by combining four different, bioengineered components produced in four different organisms 1) PSI (Thermosynechococcus elongatus), 2) HoxK/G (Ralstonia eutropha), cytochrome c553 (Escherichia coli) and PE/PC (phycoerythrin/phycocyanin) PBS (phycobilisome) rods (Synechococcus PCC 8102).
This project at the interface of science and engineering involves faculty and students from chemical and biomolecular engineering, industrial engineering, and biochemistry.
∆ back to top
Participants
PI: Bamin Khomami (CBE)
Other Info
Funding Agency: Oak Ridge National Laboratory
Amount: $60,000
Duration: 7/01/2009 - 9/31/2009
Description:
The main objective of this project is the integration of quantum mechanics determined potential parameters into atomistic molecular dynamics (MD) calculations needed to investigate actnyl-nitrate complex migration from an aqueous-organic interface to an organic phase. Specifically, we combine quantum mechanics calculations to provide an accurate description of the electrostatic interaction parameters between a uranyl-nitrate- tri-butyl-phosphate (TBP) complex and an aqueous solution.
∆ back to top
Participants
PI's: Paul Frymier (CBE), Barry Bruce (BCMB)
Other Info
Funding Agency: TN Board of Architectural and Engineering Examiners
Amount: $40,365
Duration: 1/1/2008 - 6/30/2008
Description:
In order to provide a sustainable method for using solar energy to produce a fuel, we characterize and optimize protein-metal hybrid complexes that, when exposed to light, generate hydrogen at a high rate and are temporally and thermally stable. We accomplish this by creating mutagenized complexes of cytochrome c553 and photosystem I from the thermophillic cyanobacterium Thermosynecochoccus elongatus. We recreate new residues in the native complexes to create binding sites similar to those found in green algae and higher plants. This effort is monitored by using laser flash photolysis equipment to probe the effect of genetic modification on the first two steps in the process, complex formation between cytochrome c533 and PSI and the re-reduction rate of the complex after laser-induced photooxidation of PSI.
In the flash photolysis assay, a laser is used to photo-oxidize the photosystem (photosystem I in this case). A measuring or probe beam from an arc lamp or smaller diode laser is usually used to quantify the degree of photobleaching as a function of time. In our system, we use a Spectra Physics Indi-10 Nd:YAG laser with the first harmonic frequency doubler to provide light at 532 nanometers to excite photosystem I. Our measuring beam is provided by a 150W Xenon arc lamp, appropriately filtered through a monochromater (Newport Corporation Cornerstone 260) and directed through a series of lenses through the sample. Downstream of the sample, the light passes through another monochromator and to a photomultiplier tube (Oriel Model 77361, 400-1100nm, 800nm Peak Wavelength). The signal is amplified with a transampedence amplifier (Newport 70723, DC-300 MHz, 30 dB gain) and captured on a digital oscilloscope (Tectronix) for transfer to data storage and analysis.
∆ back to top